Residual Solvent Analysis in Pharmaceuticals: A Faster, Smarter Alternative to Gas Chromatography
Residual solvent analysis is a critical quality-control step in pharmaceutical manufacturing. Detecting and quantifying trace solvents ensures product safety, regulatory compliance, and batch consistency. But traditional gas chromatography (GC) workflows can slow down development and consume valuable resources. Molecular Rotational Resonance (MRR) spectroscopy, powered by BrightSpec’s isoMRR™ platform, offers a faster, more selective path forward.
What is residual solvent analysis, and why does it matter?
Definition: Residual solvent analysis measures small amounts of solvents that remain after synthesis or purification. These solvents can affect drug safety and stability if not properly controlled.
Detail: Regulatory agencies such as the ICH classify residual solvents (Class 1–3) based on toxicity. Class 2 solvents—like methanol, acetonitrile, and nitromethane—must be monitored at ppm levels.
Example: Early detection of Class 2 residuals can shorten development cycles by identifying formulation or drying issues before scale-up.
What challenges exist with traditional gas chromatography?
Definition: Gas chromatography separates compounds by volatility, then quantifies them using detectors such as FID or MS.
Detail: While robust, GC methods require time-consuming optimization, column conditioning, and frequent recalibration.
- Long method-development cycles (often weeks)
- High consumable costs
- Limited ability to differentiate isomers
Example: A single solvent screen for five analytes might take multiple GC runs and extensive sample prep, delaying process decisions.
How does Molecular Rotational Resonance (MRR) work?
Definition: MRR measures the rotational transitions of gas-phase molecules when excited by microwave radiation.
Detail: Each molecule has a unique rotational fingerprint based on its 3-D structure. In headspace-MRR, analytes volatilize and pass into a vacuum chamber, where they are rotationally cooled (~1 K) and excited by microwaves. The emitted signal produces a high-resolution spectrum.
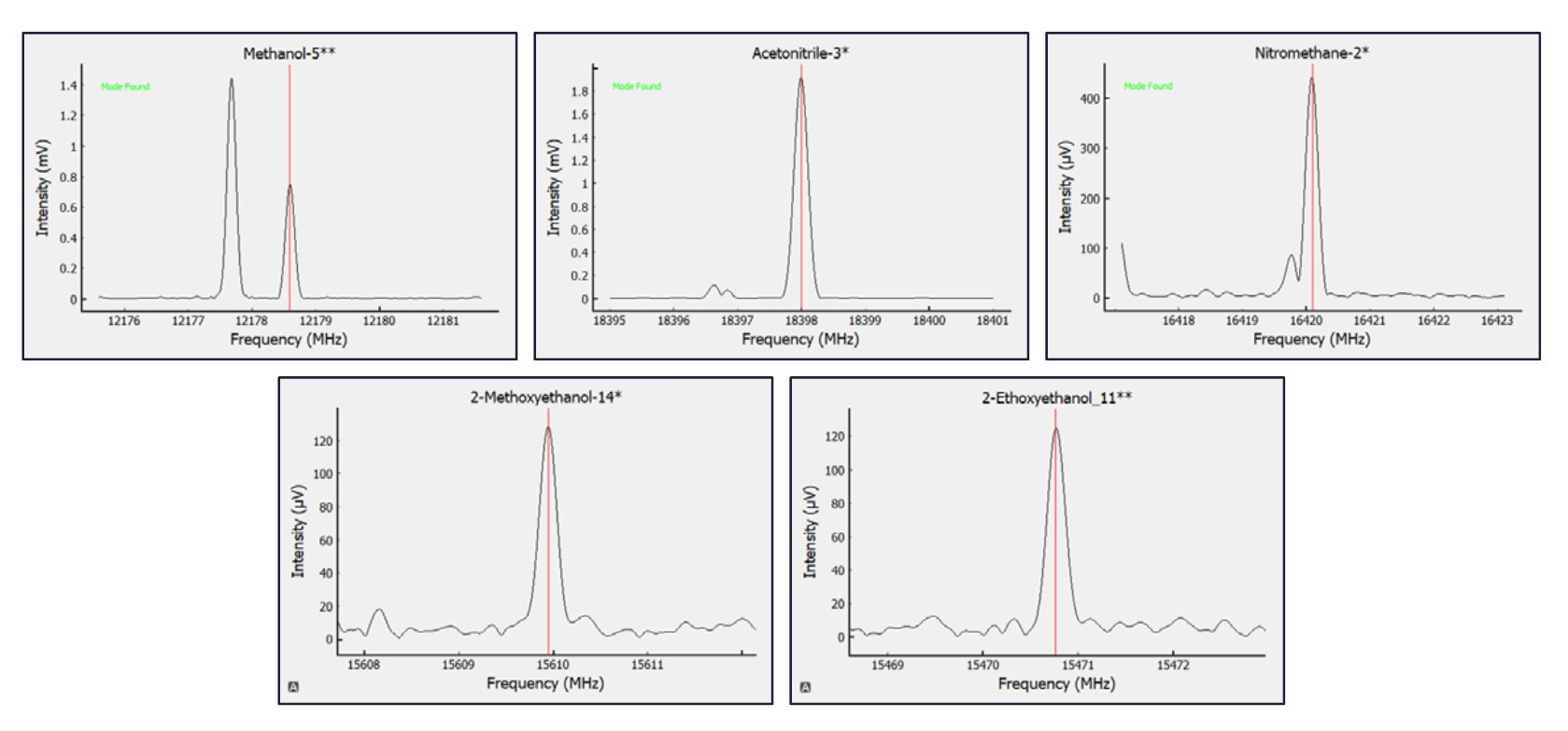
Example: Methanol peaks near 12,179 MHz while acetonitrile peaks near 18,398 MHz—distinct frequencies that allow clear identification without chromatographic separation.

What makes the isoMRR™ platform different?
Definition: The isoMRR™ platform is BrightSpec’s first commercially available MRR spectrometer optimized for residual solvent analysis.
Detail: It merges the structural specificity of NMR with the speed of MS—offering direct quantitation in a single measurement. No derivatization or consumables are required.
- Rapid analysis—results in minutes, not days
- No columns
- Automated method transfer between labs
Example: A single isoMRR™ run can simultaneously identify and quantify methanol, acetonitrile, and nitromethane in a formulation sample.
How precise and repeatable are isoMRR™ measurements?
Definition: Precision reflects how consistently the instrument measures the same sample. Linearity shows how well signal intensity correlates with concentration.
Detail: BrightSpec validated isoMRR™ using five Class 2 solvents across multiple drug matrices. Results demonstrated excellent linearity (R² ≥ 0.98) and low repeatability.
| Solvent | Linear Range (µg/mL) | R² | Repeatability (%) |
|---|---|---|---|
| Methanol | 0 – 150 | 0.997 | 6.1 |
| Acetonitrile | 0 – 20.5 | 0.992 | 11.9 |
| Nitromethane | 0 – 2.5 | 0.998 | 9.2 |
| 2-Methoxyethanol | 0 – 2.5 | 0.983 | 13.9 |
| 2-Ethoxyethanol | 0 – 8 | 0.989 | 19.0 |
Example: Methanol showed the highest precision (6 % RSD) across replicate runs, confirming reliable quantitation even at low concentrations.
How does isoMRR™ perform in real drug-substance matrices?
Definition: Recovery testing evaluates how accurately a method measures known analyte concentrations in real samples.
Detail: isoMRR™ achieved near-quantitative recovery (≈ 100 %) for methanol, acetonitrile, and 2-methoxyethanol in acetaminophen, aspirin, and ibuprofen matrices—proving matrix independence.
Example: Nitromethane recovery ranged 79–86 %, still within acceptable analytical limits.
What are the advantages of adopting MRR for residual solvent testing?
Definition: Replacing GC with MRR enhances agility and sustainability in QC labs.
Detail: MRR reduces consumables, accelerates data turnaround, and improves selectivity for challenging low-volatility solvents.
- Rapid, direct quantitation—no separation required
- Lower cost of ownership and reduced environmental footprint
- Simplified method transfer between sites
- Comprehensive solvent library verified by BrightSpec
Example: The isoMRR™ workflow enables multi-component screening in minutes, freeing analysts to focus on decision-making instead of instrument setup.
When should labs consider switching to isoMRR™?
Definition: isoMRR™ is ideal when speed, selectivity, and flexibility outweigh the need for separation.
Detail: Use MRR when developing or validating formulations containing multiple Class 2 solvents, or when GC throughput limits productivity.
- Early process development to shorten timelines
- QC release testing with high sample volumes
- Cross-lab method harmonization
Conclusion: How MRR redefines residual solvent analysis
BrightSpec’s isoMRR™ platform delivers a rapid, accurate, and chromatography-free approach to residual solvent testing. With exceptional linearity, precision, and recovery across diverse matrices, MRR spectroscopy provides the agility modern pharmaceutical workflows demand—helping teams move from method development to confident decisions faster.
Frequently Asked Questions
What is residual solvent analysis?
Residual solvent analysis measures trace solvents that remain after pharmaceutical manufacturing. Accurate detection ensures patient safety and compliance with ICH guidelines.
Why replace gas chromatography for solvent testing?
While GC is standard, it requires long method development and consumables. BrightSpec’s isoMRR platform uses MRR spectroscopy to provide direct, selective quantitation without columns.
How accurate is isoMRR for residual solvents?
isoMRR delivers excellent linearity (R² ≈ 0.997) and repeatability as low as 6 %, verified across methanol, acetonitrile, nitromethane, and other Class 2 solvents.
Which drug substances were tested?
MRR measurements were validated in acetaminophen, aspirin, and ibuprofen matrices with recoveries near 100 %, confirming accuracy in real-world formulations.
Where can I learn more?
Read the full application note or explore the USP Stimuli article on direct analysis of residual solvents using MRR.